Five Bedtime Stories
A is for Aspartame……”Oh no, We did Aspartame years ago“!
The aspartame arguments have been going on for 50 years, and suddenly, in July 2023, the WHO have raked over the embers again and the other day we picked this up in the news from Reuters, reporting on the latest WHO output…Exclusive: WHO’s cancer research agency to say aspartame sweetener a possible carcinogen
It seems the WHO cancer specialists IARC (International Agency for Research on Cancer) has categorized aspartame as “possibly carcinogenic to humans” for the first time.
These IARC definitions are ranked as follows, i)-not classifiable, ii)-possibly carcinogenic, iii)-probably carcinogenic, and iiii)-carcinogenic, all of which are based solely on weight of evidence rather than the reality of how dangerous a substance really is.
The FDA approved Aspartame in stages, originally in 1981 it was approved to add into dry foods, in 1983 it was OK in carbonated beverages, and a while after that it was give approval to add to human drug products. In 1985 Searle was bought out by Monsanto, and a decade later aspartame was finally extended for use as a general sweetener.
Aspartame, brand Name NutraSweet, was developed by G D Searle back in the 70’s, after Saccharin, the previous worlds favourite sweetener, was banned in the US when it was found to have health issues in rats, specifically tumours in their bladders. Artificial sweeteners were deemed necessary as the consumption of sugar was thought to be fattening, and a major contributor to obesity and as a result these brands were sold as diet sweeteners.
The FDA received over 7000 complaints from consumers, in one of the most contested approvals in FDA history. Time and time again the FDA have been asked to review the actual safety profile of Aspartame and each time they had refused.
Objections raised to the 1974 approval by the FDA made it necessary to conduct a PBOI (Public Board of Inquiry) to review the scientific evidence. A Searle Investigation Task Force was convened and preliminary findings claimed Searle had engaged in “dubious laboratory practices”. The investigation was completed in 1976, and just for starters: –
“FDA Toxicologist and Task Force member Dr. Adrian Gross reported that in some of the aspartame studies he reviewed, Searle lied and they didn’t submit the real nature of their observations because had they done that it is more than likely that a great number of these studies would have been rejected.”
We do not intend to wade through the whole sordid story of how Aspartame was passed fit for consumption because it is simply repeating work that has already been done by far brighter people than myself.
The full 60 minutes version of Mike Wallace on the subject is here
 Dr Ralph Walton was briefly interviewed on that 60 minutes production and circumstances conspired to alter the time he had available, and what he managed to say. There is another interview with Dr Ralph Watson in which he explains those circumstances and gives us the insights he planned to divulge on 60 Minutes. That full version is here and it contains the 60 Minute documentary. The end of that segment is at 41’28”, at which time Dr Walton begins his description of how it is possible to bamboozle regulatory offices by creating what appears to be a vast library of studies from a mere handful of data.
Dr Ralph Walton was briefly interviewed on that 60 minutes production and circumstances conspired to alter the time he had available, and what he managed to say. There is another interview with Dr Ralph Watson in which he explains those circumstances and gives us the insights he planned to divulge on 60 Minutes. That full version is here and it contains the 60 Minute documentary. The end of that segment is at 41’28”, at which time Dr Walton begins his description of how it is possible to bamboozle regulatory offices by creating what appears to be a vast library of studies from a mere handful of data.
I thought I had seen most of the Modus operandi in the peer reviewed study sphere, but this one is a new one on me.
He describes the method in the film, and he has the evidence of what appears to be a monumental fraud, but we have no way of confirming that at this time. I have just ordered his latest book and hopefully that will shed some light on it.
Also in the film he walks through the process of how the damage occurs in our bodies, which he describes as “essentially pickling ourselves”!
The next link is piece from Harvard College piece that I came across in my travels, listed as a “Historical analysis of the FDA’s decision to approve Aspartame”. Love this title…Let Them Eat Cake? A Historical Analysis of FDA’s Decision to Approve Aspartame
It does a decent job of putting the timeline together, but then,…..I got to the very end of it and read this…..
“Many consumers might not understand what a 50 mg/kg ADI really means; however, with simultaneous national education efforts, a labelling requirement would allow the consumer to conduct a private risk-benefit analysis of his own. With hysterical and confused consumers in one corner, diabetics and the weight-conscious unwilling to surrender their delicious sweetener in another, and science hovering uncertain and splintered in the middle, shifting the information and responsibility to the individual might be the most pragmatic, and conscionable, step FDA could take.
So…
- We are too thick to understand what a 50mg/kg ADI means. (ever heard of Google?)
- We are thought of as hysterical and confused.
- The diabetics and weight conscious are unwilling to give up their sweetener ?????
- The poor old scientists are splintered and uncertain.
- Because of all this confusion its best left for the individual (you and I) to shoulder the responsibility by taking it off the shoulders of the poor old FDA, (for which they get very well paid,)but are unfortunately uncertain and splintered right now.
And now you know as much as I do on the subject of Aspartame, including how the next generation of movers and shakers that graduate from Harvard think about the general public at large…
More on this subject next week.
B is for B*m (as in “Smooth as a Babys…”)
The New York Times reported in April 2023 …
“Johnson & Johnson said on Tuesday that it had agreed to pay $8.9 billion to tens of thousands of people who claimed the company’s talcum powder products caused cancer, a proposal that lawyers for the plaintiffs called a “significant victory” in a legal fight that has lasted more than a decade.”
Talcum powder is a clay mineral compound of Magnesium Silicate and is often used in cosmetics due to its very fine structure.
Talc is mined in areas that are very close to the deposits of asbestos, and from a chemical perspective they are often mixed together, and nigh on impossible to separate.
This has been known to the mining industry for decades, and as it turns out, J & J knew about it for decades too.
From that linked Reuters article:-
J&J didn’t tell the FDA that at least three tests by three different labs from 1972 to 1975 had found asbestos in its talc – in one case at levels reported as “rather high.”
Internal records from J & J showed that they knew about possible contamination back in 1957/8, and chose to bury it. They certainly didn’t tell the FDA about that either. This damning evidence, as is often the case, only came to light when specific documents were demanded by the court. It is therefore possible that this decades long evasive action contributed to the slow and painful deaths of thousands of people.
In October 2021, a spin-off from J & J, called LTL management, apparently set up to absorb the liabilities of the Asbestos Talc claims, filed for bankruptcy protection.
On the 7th July 2023, that same LTL Management filed a complaint against 3 of the doctors who had published material making the original claims of a linkage between the talcum powder and various forms of cancer.
I somehow doubt that the $8.9 Billion will have been handed over to the claimants as yet, and should that turn out to be true, it looks like they will have another nice long wait for their justice at the hands of the US legal profession. Another nice business to do people with.
C is for Chemical nightmare
D is for Definition,
{or not as the case may be}
This is the abstract from that study:-


As a “Non-Scientist” I have a couple of observations on this table…
- Out of the nine definitions studied, the first two do not apply, at the time of the regulation no such material had been developed.
- 2 of the definitions (ringed in red) had neither ethical nor scientific justifications for their existence.
- One of the remaining 5 (FDA) states that GTP regulatory scrutiny does not apply to vaccines. (This scrutiny is apparently much stricter than the regulation of vaccines which in the case of Covid 19 vaccines, were targeted at the entire population of the world).
- EU Directive 2003 and EU directive 2009 seem to conflict with each other, the first one makes no mention of vaccines but puts products “with a piece of nucleic acid” in the same category, including vaccines by default (the RA in mRNA IS Nucleic Acid). The second directive excludes vaccines from the GTP regulatory process without “Ethical or scientific justification”.
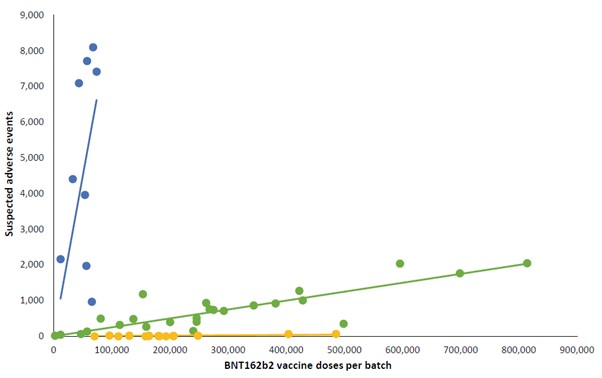
E is for Everybody (YKWIM)
- A total of 10,793,766 doses were administered to…
- 4,026,575 persons
- 52 Batches of vaccine were used to do it.
- A total of 66,587 Suspected Adverse Events were recorded
- 7.11% of the SAE’s had an incomplete record of the vaccine batch numbers attached.
- Leaving 61,847 Batch identifiable SAE’s, of which 14,509 were classified as serious and 579 were fatal.